
These may include spinal block of varying magnitude including total spinal block hypotension secondary to spinal block loss of bladder and bowel control and loss of perineal sensation and sexual function. Hepatic effects ranging from slight elevations in liver enzymes to rare cases of hepatic failure have been reported see ADVERSE REACTIONS and PRECAUTIONS Laboratory Tests.
Allergic reactions can develop to tetrazepam and it is considered to be a potential allergen.
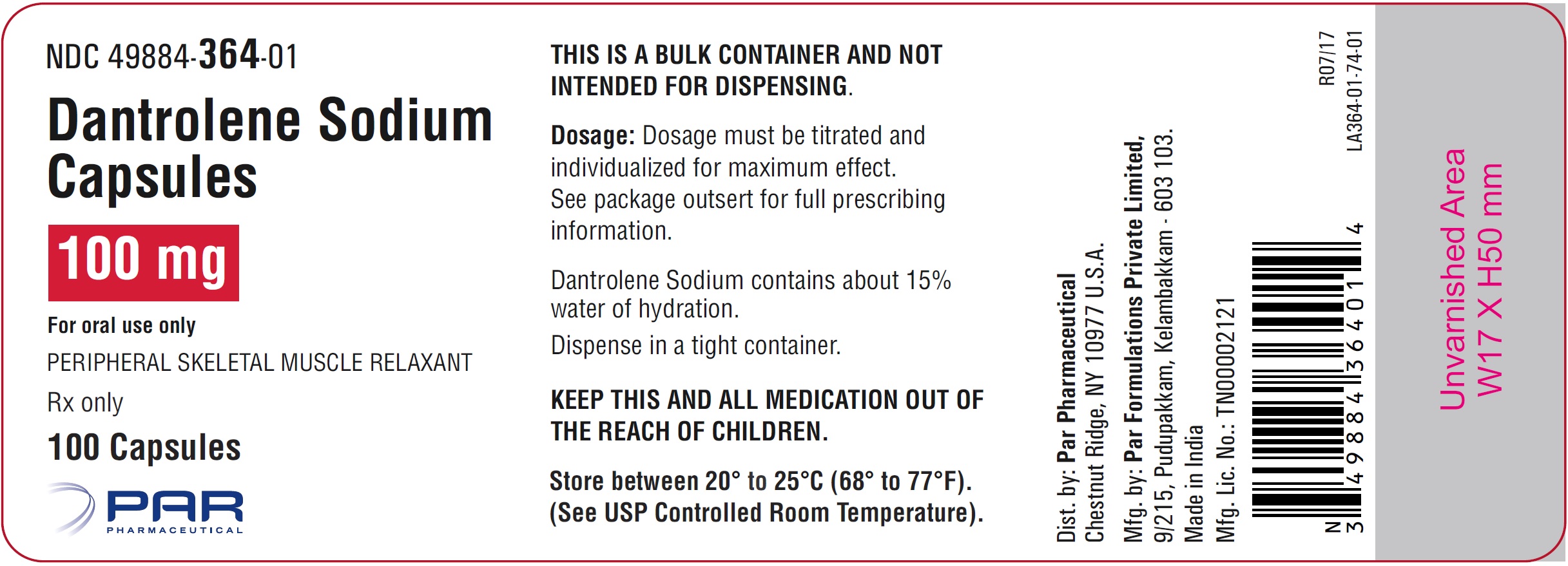
Dantrolene adverse effects. Dantrolene sodium sold under the brand name Dantrium among others. Central nervous system side effects are quite frequently noted and encompass speech and visual disturbances mental depression and confusion hallucinations headache insomnia and exacerbation or precipitation of seizures and increased nervousness. Infrequent cases of respiratory depression or a feeling.
Dantrolene may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away. In addition to the symptoms mentioned in the IMPORTANT WARNING section if you experience and of the following symptoms call your doctor immediately.
Difficulty breathing or slow shallow breathing. If you experience a serious. Dantrolene sodium is a postsynaptic muscle relaxant with multiple indications in the fields of anesthesiology and neurology.
While there are many indications for the use of dantrolene its primary indication and FDA-approved usage in both children and adults is for the treatment of malignant hyperthermia. The very rare but life-threatening disorder triggered by general anesthesia. The adverse effects of antipsychotic medications range from relatively minor tolerability issues eg mild sedation or dry mouth to very unpleasant eg constipation akathisia sexual dysfunction to painful eg acute dystonias to disfiguring eg weight gain tardive dyskinesia to life threatening eg myocarditis agranulocytosis.
Some adverse effects have little shortterm. Limited information indicates that oral baclofen to the lactating mother appears in low levels in milk and would not be expected to cause any adverse effects in breastfed infants especially if the infant is older than 2 months. Monitor newborn infants for signs of sedation.
Low intrathecal doses and topical application are unlikely to affect the nursing infant. In a woman who received a. Dantrolene and codeine both increase sedation.
Darifenacin decreases effects of codeine by affecting hepatic enzyme CYP2D6 metabolism. Prevents conversion of codeine to its active metabolite morphine. Desflurane and codeine both increase sedation.
Opioids may decrease MAC. Dantrolene and doxepin both increase sedation. Darifenacin will increase the level or effect of doxepin by affecting hepatic enzyme CYP2D6 metabolism.
Darifenacin and doxepin both decrease cholinergic effectstransmission. Doxepin and dasatinib both increase QTc interval. Moderate General anesthetics potentiate the effects of other CNS depressants.
Major Adverse cardiovascular effects can develop as a result of concomitant administration of oxytocin with general anesthetics especially in those with preexisting valvular heart disease. Cyclopropane when administered with or without oxytocin has been implicated in producing. For ratings users were asked how effective they found the medicine while considering positiveadverse effects and ease of.
Allergic reactions to tetrazepam occasionally occur involving the skin. Allergic reactions can develop to tetrazepam and it is considered to be a potential allergen. Drug rash and drug-induced eosinophilia with systemic symptoms is a known complication of tetrazepam exposure.
Subsequent adverse effects may depend partially on the amount of drug administered subdurally. These may include spinal block of varying magnitude including total spinal block hypotension secondary to spinal block loss of bladder and bowel control and loss of perineal sensation and sexual function. Persistent motor sensory andor autonomic sphincter control deficit of some.
Hepatic effects ranging from slight elevations in liver enzymes to rare cases of hepatic failure have been reported see ADVERSE REACTIONS and PRECAUTIONS Laboratory Tests. In some cases hepatic effects may progress despite discontinuation of the drug. In addition rare instances of vanishing bile duct syndrome have been reported.
Adverse effects include hypotension bradycardia and myocardial depression. Dexmedetomidine Precedex A. Indicated for sedation and treatment of anxiety of initially intubated and mechanically ventilated patients during treatment in an intensive care setting.
It should be administered by continuous infusion not to exceed 24 hours. A specific and selective α2-adrenergic agonist.